and the distribution of digital products.
Strategic Choices for AI in Biopharma: Partnering for Effective Implementation
As the pharmaceutical industry evolves, Generative AI (GenAI) is becoming a vital tool for optimizing operations, expediting regulatory processes and driving innovation. With rising regulatory demands and competitive pressures to accelerate drug development, biopharma companies are increasingly turning to collaborative AI platforms with built-in compliance and adaptability.
Biopharma companies now face a critical decision: build an in-house AI solution or invest in an external platform. This decision impacts more than technology—it also affects costs, time to market, compliance and scalability. This blog explores how a proven GenAI solution may offer a more effective, sustainable path forward for biopharma.
The Reality of Generative AI in Regulatory Writing
One of the most impactful applications of generative AI in biopharma is in regulatory writing, where speed and accuracy are paramount. Generative AI is transforming regulatory documentation by turning vast datasets into actionable insights, speeding up drug development and simplifying tasks like Clinical Study Reports (CSRs) and Patient Narratives. Generative AI can automatically draft patient narratives based on structured clinical data, a process that traditionally requires hours of manual writing. These documents are crucial for regulatory approval and GenAI’s ability to automate and maintain accuracy makes it an invaluable asset. However, the complexity and costs of building and maintaining in-house GenAI solutions are often underestimated.
Advanced AI capabilities, especially those comparable to industry leaders, require continuous investment and specialized knowledge. In biopharma, data governance and compliance add to these demands. Developing in-house solutions can be further complicated in regulated environments, where maintaining an up-to-date system requires ongoing resources.
Tim Martin, Executive VP of Product & Development at Yseop: “In biopharma, the ‘build vs. buy’ decision for AI is ultimately about focus—do you want to be an AI developer or an innovator in drug development? By partnering with a proven GenAI provider, companies can streamline regulatory processes, enhance scalability and drive innovation without the burden of maintaining complex infrastructure.”

Challenges of In-House Development
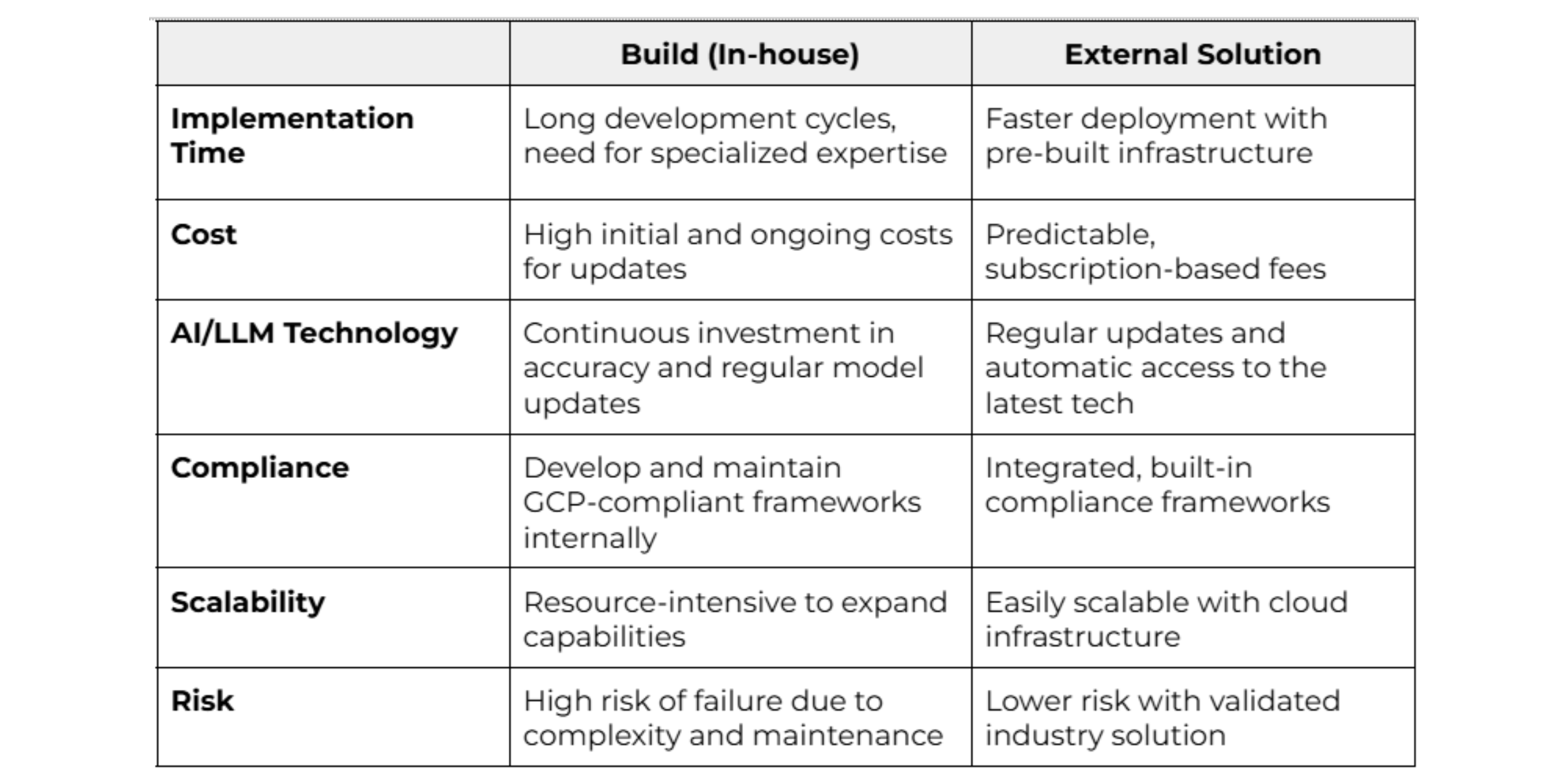
- Implementation Time: Developing a GenAI solution requires specialized expertise and complex integration, especially when data is dispersed across departments. This often demands significant investments to ensure seamless accessibility and alignment.
- Unpredictable Costs: In-house AI requires high initial and ongoing investments in infrastructure and specialized talent, often exceeding initial projections. Subscription-based models, in contrast, offer more predictable and sustainable costs.
- Data Privacy, Compliance & Maintenance: Ensuring continuous compliance with standards like GCP and ICH demands ongoing resources and can strain internal teams.
Key Considerations: Build vs. External Solution
In biopharma, choosing between custom in-house solutions and proven external platforms means balancing flexibility with rapid deployment and compliance. External solutions also offer future-proofing, keeping pace with AI advancements to reduce reinvestment needs. Here are a few things to consider when making your decision.
Finding the Right Path to Innovation
Generative AI is no longer just a concept for the future; it is actively reshaping biopharma operations today. For companies aiming to leverage this technology effectively, choosing the right AI partner is essential to truly reduce costs, accelerate time to market and maintain regulatory compliance. By adopting a scalable, compliant solution from a trusted provider, biopharma companies benefit not only cutting-edge tools but also the assurance that their technology evolves in step with industry advancements.
In a field where the primary mission is drug development and patient care, partnering with a dedicated AI software provider allows biopharma companies to focus on what they do best—delivering innovative therapies. Rather than undertaking the resource-intensive task of building AI solutions internally, companies can rely on a partner equipped with the expertise and infrastructure needed to address industry-specific challenges, streamline workflows and foster impactful innovation.
Working with an AI partner ensures that biopharma companies stay at the forefront of technological progress, empowering them to advance toward their goals with confidence.
The post Strategic Choices for AI in Biopharma: Partnering for Effective Implementation appeared first on Yseop.
- Home
- About Us
- Write For Us / Submit Content
- Advertising And Affiliates
- Feeds And Syndication
- Contact Us
- Login
- Privacy
All Rights Reserved. Copyright , Central Coast Communications, Inc.