and the distribution of digital products.
Evaluating the Economic Viability of Silver and Lead-Based Photocatalysts
:::info (1) Sean M. Stafford, Department of Chemical Engineering and Materials Science, Michigan State University, East Lansing, MI, 48824, USA;
(2) Alexander Aduenko, Moscow Institute of Physics and Technology, Moscow, Russia;
(3) Marcus Djokic, Department of Chemical Engineering and Materials Science, Michigan State University, East Lansing, MI, 48824, USA;
(4) Yu-Hsiu Lin, Department of Chemical Engineering and Materials Science, Michigan State University, East Lansing, MI, 48824, USA;
(5) Jose L. Mendoza-Cortes, Department of Chemical Engineering and Materials Science, Michigan State University, East Lansing, MI, 48824, USA (Email: [email protected]).
:::
Table of LinksSALSA- (S)ubstitution, (A)pproximation, Evo(L)utionary (S)earch, and (A)B-Initio Calculations
SALSA Applied to Photocatalytic Water-splitting
Conclusions, Data Availability Statement and References
Appendix: Supplementary Material
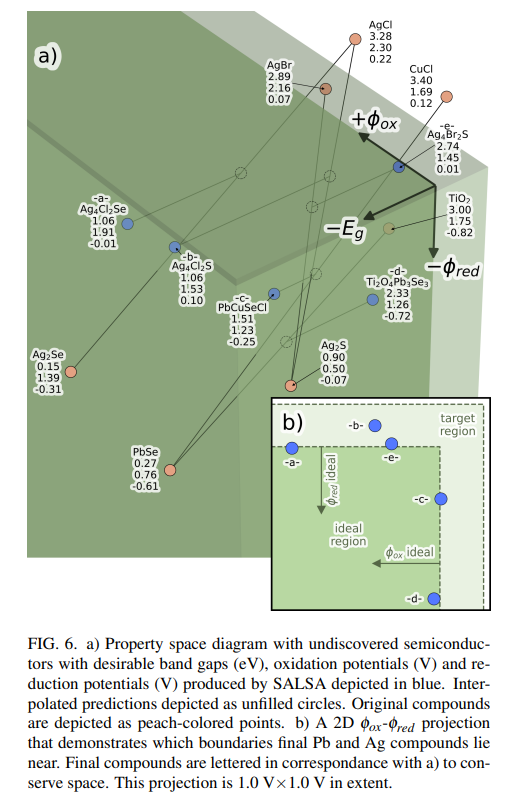
IV. DISCUSSIONFigure 6 (a) presents the interpolations into property space from our initial compounds which yielded our final structures, as well as shifts from interpolated predictions. Trends within this subset of interpolations suggest certain paths are favored for producing a photocatalytic water-splitter.
\ Our final materials can be divided into two groups. One group is made up of materials containing Silver, halides and group 16 compounds. Among the few compounds in our initial dataset which had good oxidation potentials, most contained Silver, so this group emerged from the interpolation between a pair of materials which had good oxidation potentials, but which had band gaps that were too low and too high respectively. Consequently, these materials are robust to hole oxidation – all interpolated oxidation potentials are at least 0.2 V greater than the ideal minimum. However, their interpolated reduction potentials lie close to the threshold for rejection – none are more than 0.01 V under the ideal maximum. Additionally, these structures have low symmetry and are expensive due to their Silver content.
\ The other group contains Lead instead of Silver. Redox suitability of these Lead compounds is inverted relative to the Silver group. That is, these compounds are robust to electron reduction due to Lead’s especially negative reduction potential – all have reduction potentials more than 0.2 V under the ideal maximum. However, none have an interpolated oxidation potential that is 0.03 V greater than the ideal minimum. The Lead structures are also higher in symmetry and relatively cheap. Figure 6 (b) highlights how compounds from different groups lie near different planes of the desired property space, demonstrating the strengths and weaknesses of these groups.
\ The Lead group is about 50 times cheaper so it may offer more scalability.27–31 However, the Silver group follows more closely to regular compositional formula. This means it may be easier to find more compounds in this group with different interpolation ratios if the ones we have discovered do not prove to be as effective as they appear to be. Both paths should be investigated experimentally.
\ Furthermore, we envision the materials design approach we used to be generalizable. In a different scheme, we would see a picture similar to this, but with different starting compounds and different boundaries than our target property space.
\
\
:::info This paper is available on arxiv under CC 4.0 license.
:::
\
- Home
- About Us
- Write For Us / Submit Content
- Advertising And Affiliates
- Feeds And Syndication
- Contact Us
- Login
- Privacy
All Rights Reserved. Copyright , Central Coast Communications, Inc.